Tackling the impairment of the protein quality control systems in neurodegenerative disorders: from bench to bedside
What we know
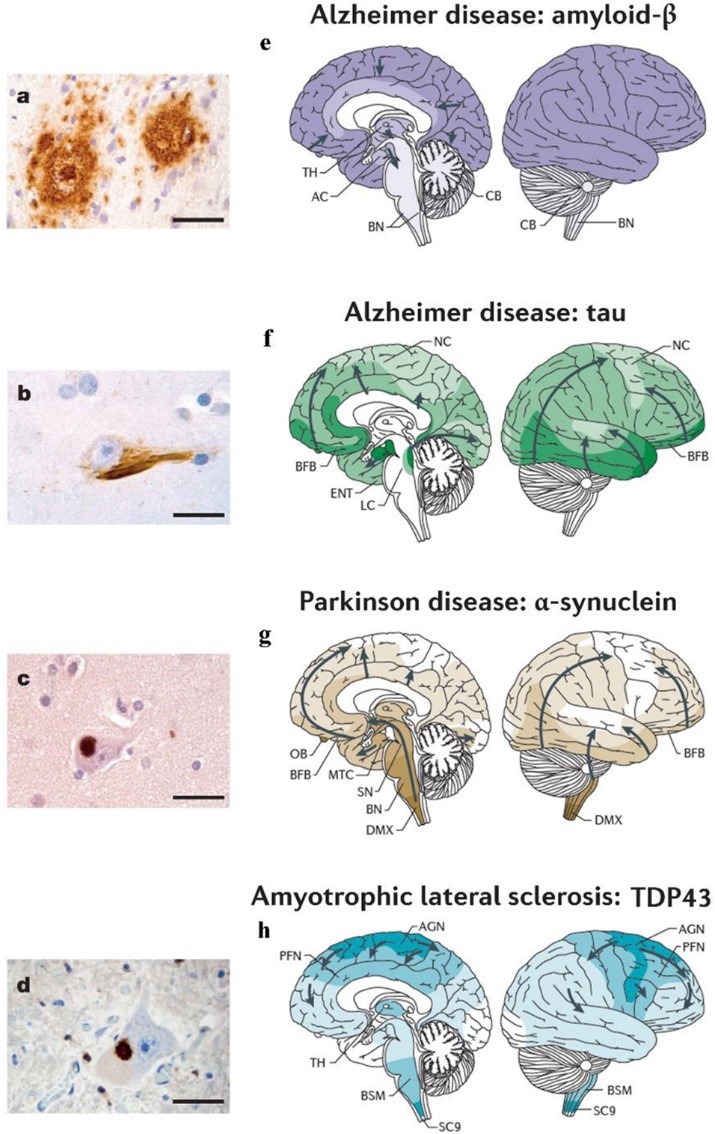
Many neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS), belong to the large category of proteinopathies, conditions characterized by the presence of proteinaceous inclusions within and/or outside the degenerating neurons.
(Carbonell et al., Front. Neurol. 2018)
The identification of such aggregates supports the view that misfolded proteins represent a basic requirement for the neurodegenerative process and provided input to verify the existence of possible dysfunctions of the biological systems influencing neuronal protein homeostasis.
Considering the post-mitotic nature of neurons, a proper activity of the intracellular protein degradation systems appears to be crucial to ensure the maintenance of cell homeostasis and to prevent the onset of the neurodegeneration.
The ubiquitin-proteasome system (UPS) and the autophagy-lysosome pathway (ALP) are the two major protein catabolic systems that cooperate in maintaining the protein quality control. Alterations of both UPS and ALP have been reported in patients with AD, PD and ALS, supporting a role for protein quality control system dysfunction in neurodegeneration.
Future perspectives
We are currently using different pharmacological or genetic in vitro models of neurodegenerative diseases to study the molecular mechanisms involved in ALP dysfunction and to identify new possible neuroprotective compounds. The deriving results could lead to a better understanding of the pathogenesis of neurodegenerative diseases and to the identification of new therapeutic targets.
The in vitro study is paralleled by an ex vivo study in cells obtained from patients (lymphomonocytes or fibroblasts) aimed at investigating the existence of possible systemic autophagy alterations useful to identify new peripheral disease biomarkers for early diagnosis, personalized therapy, or monitoring of drug efficacy in clinical trials.




