Lab Experience Personal reflection – Gaia Giulietti
Lab Experience Personal reflection
Gaia Giulietti, Virgilio 4th Cohort Student – Humanitas University
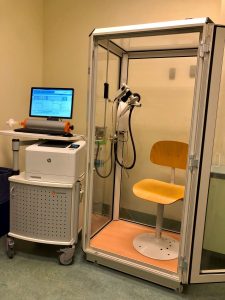
Laboratory: Personalized medicine, asthma and allergy unit
PI of the laboratory: Prof. Giorgio Walter Canonica
Tutors: Prof. Enrico Heffler and Prof. Giovanni Paoletti
In September 2021 I was given the opportunity to do my first lab rotation in the Personalized medicine, asthma and allergy unit at Humanitas Research Hospital.
The unit is coordinated by Professor G.W. Canonica, former head of the SIAAIC (Società Italiana di Allergia, Asma e Immunologia Clinica) and former head of the WAO (World Allergy Organization).
The team is dynamic, and different randomized clinical trials were carried out simultaneously focusing mostly on severe asthma, nasal polyposis, chronic urticaria, mastocytosis and eosinophilic esophagitis.
In particular, the main clinical trials I took part into were NOVARTIS, NAPREB, Dupilumab MAC and Messina Project.
- NOVARTIS is a project based on the administration of inhaled anti-TLSP in patients with controlled asthma (GINA level 3-4).
- NAPREB is a study which uses the monoclonal antibody Benralizumab (anti-IL5) in order to treat nasal polyposis.
- Dupilumab is an anti-IL4 and anti-IL13 biological drug used for the treatment of both severe asthma and nasal polyposis. In the case of nasal polyposis, patients were first assessed by otorhinolaryngologists and then regularly monitored by the unit via spirometry, bronchodilation test, complete FeNO (up to 350ug/ml) and nasal FeNO.
- Messina project is a study funded by AstraZeneca with the aim of verifying the efficacy of Benralizumab (anti IL-5) in the treatment of eosinophilic esophagitis. Eosinophilic esophagitis is a chronic immune system disease where eosinophils build up in the esophagus. This build up can inflame or injure the esophageal tissue, which in turn can lead to difficulties in swallowing or cause food to get stuck when swallowing. The study is characterized by 15 sessions (one each month), during which the response of the patient to therapy is monitored. This last project also gave me the opportunity to examine esophageal endoscopic reports and collaborate with gastroenterologists.
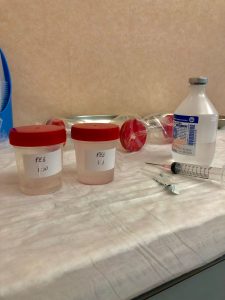 My tutors followed me through every step of the trials and carefully explained to me how and why specific tests were carried out. This gave me the opportunity to fully understand the basics behind each clinical trial.
My tutors followed me through every step of the trials and carefully explained to me how and why specific tests were carried out. This gave me the opportunity to fully understand the basics behind each clinical trial.
I also learnt how even the smallest bias may lead to “screen failure”. In these cases, the team was now discouraged, but kept moving forward with the same passion and strength.
Thanks to this experience I truly realized how biological drugs practically influence the quality of life of patients. I’ll never forget how happy the patients were after they experienced the benefits of monoclonal antibodies and how they were able to go back to their normal lives.
As a conclusion, I can say with extreme confidence that this experience taught a lot not only from a scientific point of view, but also from a human perspective.
I am really looking forward to beginning a new chapter!




